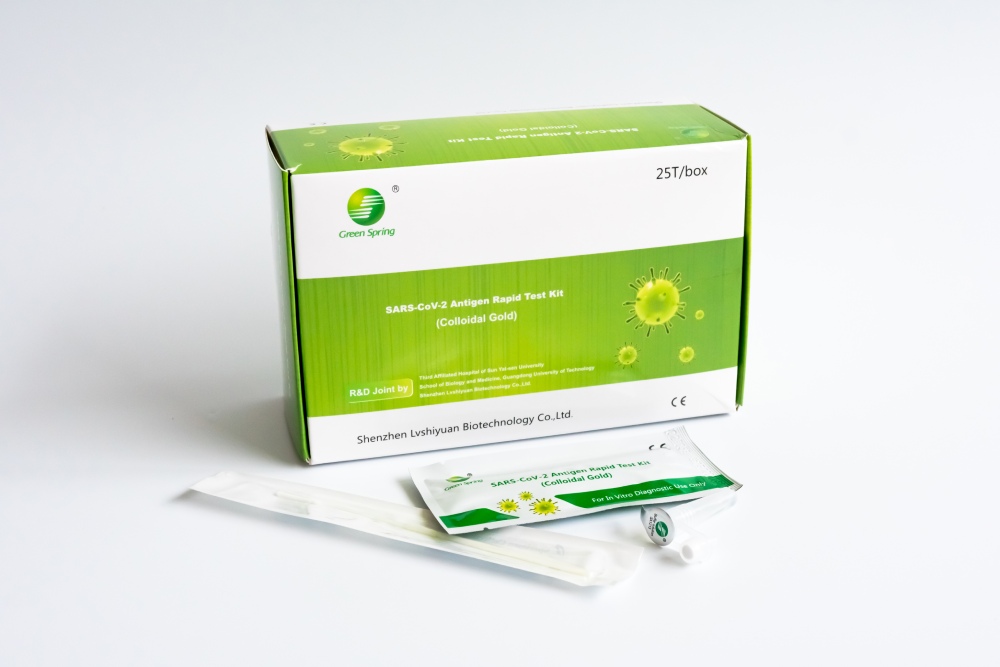
Greenspring – COVID-19 Professional Rapid Test Kits
15,50 €*
Available, delivery time 5 - 7 Working days
Buy Green Spring SARS-CoV-2 Antigen Rapid Test Kit - Green Spring Rapid Test 4 in 1!
Detect COVID-19 infections quickly and accurately with our Green Spring SARS-CoV-2 Antigen Rapid Test Kit. The high sensitivity of 96.77% and 100% specificity ensure high-quality performance.
The 4 sampling options (nasopharyngeal, oropharyngeal, nasal, and saliva) allow for flexible and individual sample collection.
Features of the Green Spring SARS-CoV-2 Antigen Rapid Test Kit
✓ Listed on the Common RAT List of the HSC
✓ Test result after 15 minutes
✓ Can be performed flexibly and independently of location
✓ Only for medical professionals
✓ Listed on the positive list of the Paul-Ehrlich-Institut
✓ BfArM listed, Test-ID: AT417/20
All of our tests are CE certified and suitable for use in the European market.
What's included in the Green Spring SARS-CoV-2 Antigen Rapid Test Kit 4 in 1
25 sterile swabs
25 test cassettes
25 extraction tubes
1 tube stand
1 user manual
Order now and detect COVID-19 quickly, reliably, and easily with the Green Spring SARS-CoV-2 Antigen Rapid Test Kit - Green Spring Rapid Test!
Why Buy the Green Spring SARS-CoV-2 Antigen Rapid Test Kit - Green Spring Rapid Test?
✓ MONEY-BACK GUARANTEE
Our goal is your satisfaction with your purchase. If the purchased goods do not meet your expectations, you can return them within the 14-day cancellation period and you will be refunded the purchase price.
| Comparison - Professional COVID-19 Antigen Rapid Tests | |||||||
|---|---|---|---|---|---|---|---|
| Manufactured by | Hecin | Testsealabs | Bioteke | Longsee | Green Spring | Roche | Siemens |
| Sensitivity | 97.09% | 92.10% | 96.49% | 95.51% | 96.77% | 96.52% | 97.25% |
| Specificity | 99.78% | 98.10% | 99.28% | 99.72% | 100% | 99.60% | 100% |
| Manufactured in | China | China | China | China | China | South Korea | China |
| Listed for the "EU common list" |
Yes, in category A | Yes | Yes | Yes, in category A | Yes | Yes | Yes |
Frequently Asked Questions Before Buying the Green Spring SARS-CoV-2 Antigen Rapid Test Kit
What is the Green Spring SARS-CoV-2 Antigen Rapid Test Kit?
The Green Spring SARS-CoV-2 Antigen Rapid Test Kit is a rapid COVID-19 antigen test with high specificity and sensitivity. It has been evaluated by the Paul Ehrlich Institute and differs from other rapid tests by offering 4 options for sample collection (nasal, nasopharyngeal, oropharyngeal, or saliva swab).
Does the Green Spring Rapid Test for SARS-CoV-2 Antigen have the capability to detect mutations?
Yes, the Green Spring 4-in-1 COVID-19 antigen rapid test can detect, among other variants, the Delta and Omicron variants.
Is the Green Spring Rapid Test safe?
The Green Spring SARS-CoV-2 Antigen Rapid Test Kit - Green Spring Rapid Test has been evaluated by the PEI and has an overall sensitivity of 100%. The test's sensitivity is 95.51% and specificity is 99.72%. Since the test is on the "Common List" (European Commission list), it can be assumed to be safe.
How long does it take to get the results of the Green Spring SARS-CoV-2 Antigen Rapid Test Kit?
You can read the results of the Green Spring test in just 15 minutes.
Is the Green Spring test suitable for children?
Yes, the Green Spring SARS-CoV-2 Antigen Rapid Test is more suitable for children than other tests, especially because it can be done with lollipops.
How is the professional Green Spring Rapid Test packaged?
There are 25 tests in one box, and 1000 in one whole carton.
How long is the Green Spring SARS-CoV-2 Antigen Rapid Test Kit - Green Spring Rapid Test storable?
The Green Spring SARS-CoV-2 Antigen Rapid Test can be stored at room temperature or between 4 and 35 degrees for up to 12 months.
Does the Green Spring Rapid Test detect the Omicron variant?
Yes, the tests have been evaluated by the Paul-Ehrlich-Institut. The Omicron variant is detected in full.
Which tests are on the Paul-Ehrlich-Institut's checklist?
The list includes tests that meet the sensitivity criteria and are in the top 20% of the list. You can read the exact details here.
Who is allowed to use the Green Spring Rapid Test?
These tests are exclusively intended for professional use. Home antigen tests can be found here.
Technical Data
Packing
25 packages
Cardboard
40 boxes - 1000 pieces
Standards and market conformity
Professional use (only available for professional users)
Quick test 4 in 1
Approved in Germany
EN ISO 13485:2016
B Company number: AT417/20
Listed for EU-wide recognition in the "EU Common List" of the European Commission - Directorate General Health and Food Safety.
CE-certified and reimbursable test, listed at the Federal Institute for Drugs and Medical Devices according to the Coronavirus Test Regulation (TestV)
96.77% sensitivity 100% specificity
With an integrated buffer solution
Green Spring SARS-CoV-2 Antigen Rapid Test Kit Test Instructions
Rapid test for the qualitative detection of SARS-CoV-2 nucleocapsid antigens. For professional use.
PURPOSE
The Green Spring® SARS-CoV-2 Antigen Rapid Test is for the rapid qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in human saliva, nasal, nasopharyngeal or throat swab specimens. The results are used for the detection of SARS-CoV-2 antigen. The antigen is generally detectable in upper respiratory tract specimens during the acute phase of infections. Positive results do not rule out bacterial infection or co-infection with other viruses. The pathogen detected may not be the sole cause of the disease. Negative results should be treated as suspected cases and confirmed with a molecular assay. Negative results should be considered in the context of a patient's recent exposures, history and presence of clinical signs and symptoms consistent with COVID-19. These tests should only be used by professionals or trained individuals.
SUMMARY
The novel coronaviruses belong to a ß-genus. COVID-19 is an acute respiratory infectious disease. Humans are generally susceptible to it. Currently, patients infected with the novel coronavirus are the main source of infection; asymptomatically infected people may also be a source of infection. The main manifestations include fever, fatigue and a dry cough. A stuffy or runny nose, sore throat, muscle aches and diarrhoea occur in a few cases.
TEST PRINCIPLE
The Green Spring® SARS-CoV-2 Antigen Rapid Test is a qualitative, membrane-based immunoassay for the detection of SARS-CoV-2 nucleocapsid protein antigens. The test line region is coated with SARS-CoV-2 antibody. The sample reacts with the SARS-CoV-2 antibody in the test line region. If the sample contains SARS-CoV-2 antigens, a coloured line appears in the test line area (T) as a relevant result. As a procedural control, a coloured line appears in the control line area (C), indicating that the correct volume of sample has been added and membrane wetting has occurred correctly
STORAGE AND STABILITY
Store the tests in the sealed foil pouch at room temperature or refrigerated (2 - 30 °C). The test is stable until the expiry date printed on it. The test cassettes must be stored in the sealed foil pouch until use. Do not freeze. Do not use after the expiry date. Protect from sun, moisture and heat.
MATERIALS SUPPLIED
• Test cassette with one pack of desiccant: 25 pieces
• Sterile swab: 25 pieces
• Extraction tubes with buffer: 25 disposable extraction tubes with 0.5 ml extraction buffer each and 25 nozzle caps.
• Workstation: 1 piece
• Package insert: 1 instruction leaflet
Precautionary Measures
1. The package leaflet must be read carefully before performing the test. Failure to follow the instructions in the package leaflet may lead to inaccurate test results.
2. For professional use in in vitro diagnostics only. Do not use after the expiry date.
3. Do not eat, drink or smoke for 10 minutes before and during sample collection.
4. Do not use the test if the packaging or test components are damaged.
5. All specimens must be considered potentially infectious. Observe established precautions against microbiological hazards throughout the collection, handling, storage and disposal of patient specimens and used test components.
6. Wear protective clothing such as lab coats, disposable gloves and eye protection while samples are being examined
7. Wash your hands thoroughly after performing the test.
8. Samples stored in viral transport media (VTM) may influence test results.
9. All used test components should be disposed of according to local regulations.
10. Humidity and temperature can adversely affect the results
PREPARATION
Only use the materials supplied with the respective set. Test the samples immediately
Use the test kit only at room temperature (15 to 30 °C). The test kit is intended only for swab specimens that are collected and tested directly (i.e. swabs that have NOT been placed in transport media). This kit is NOT intended for testing liquid specimens such as wash or aspirate specimens or swabs in transport media, as results may be affected by dilution.
1. Tear off the foil pouch, remove the test cassette and place it on a clean and flat surface.
2. Freshly collected samples should be processed within 1 hour.
3. Label the respective test cassette for each test or control.
4. Place the labelled extraction tubes in a rack in the designated area of the workspace.
SAMPLING
The correct sample collection is the most important step. Choose one of the four methods and then proceed with the test procedure.
1) Salvia (Lolli-Test)
Be aware that false results may occur if saliva is not collected properly.
1. Place an extraction tube in the cardboard workstation.
2. Press the tip of the tongue against the lower root of the jaw. Cough deeply. Make the sound of "kuuua" to concentrate the saliva.
3. Place the swab on the tongue for at least 10 seconds, turning it 3 times or more to fully absorb the saliva.
2) Anterio-nasal swab (nose in front)
Make sure to collect enough nasal secretions with the swab. It is advisable to blow your nose first.
1. Place an extraction tube in the cardboard workstation.
2. Carefully insert the swab into the patient's nostril. The tip of the swab should be inserted up to 2.5 cm deep from the edge of the nostril.
3. Dab along the mucous membrane in the nostril to ensure that both mucus and cells are collected.
4. Remove the swab from the nostril while gently rotating it between your fingers.
3) Nasopharyngeal swab (nose and throat)
1. Place an extraction tube in the cardboard workstation.
2. Tilt the patient's head slightly backwards. Hold the swab like a pen and insert it through the nostril parallel to the palate.
3. While inserting, gently rub and roll the swab. As soon as you feel the throat resistance, stop and let the swab absorb secretion.
4. Slowly and gently remove the swab outwards while gently rotating it between your fingers.
4) Oropharyngeal swab (throat)
1. Place an extraction tube in the cardboard workstation.
2. Have the patient open his/her mouth wide and make "Ah" sounds, exposing the pharyngeal tonsils on both sides.
3. Hold the swab firmly and wipe back and forth on the pharyngeal tonsils on both sides at least three times per side with moderate force. Do not touch the palate, tongue, teeth or gums.
4. Remove the swab while gently rotating it between your fingers.
For best results, the nasopharyngeal (nose to throat) method is recommended.
TESTING
After taking the sample, perform the test as follows:
1. Tear off the seal of the extraction buffer tube.
2. Insert the swab into the tube and dip it up and down into the liquid for at least 10 seconds. Then hold the swab against the bottom of the tube and turn it 3 times, making sure that no contents splash out of the tube.
3. Remove the swab while squeezing the sides of the tube to extract the liquid from the swab.
4. Place the dropper tip firmly on the extraction buffer tube and mix the liquid thoroughly.
5. Add 3 drops (approx. 100μL) via the dropper tip into the sample well of the test cassette.
6. Evaluate the test results after 15 minutes. Do not evaluate the results after 20 minutes.
INTERPRETATION OF THE TEST RESULT
POSITIVE: Two lines appear. One coloured line appears in the control line region (C) and another coloured line appears in the test line region (T). A positive result in the test region indicates the detection of SARS-CoV-2 antigens in the sample. A positive result does not exclude infection with other pathogens
NEGATIVE: A coloured line appears in the control area (C). No visible coloured line appears in the test line area (T). A negative result does not rule out viral infection with SARS-CoV-2 and should be confirmed by molecular diagnostic methods if COVID-19 is suspected.
INVALID: The control line does not appear. Insufficient sample volume or incorrect handling are the most likely reasons for the control line not appearing. Check the procedure and repeat the test with a new test cassette. If the problem persists, stop using the test kit immediately and contact your dealer.
QUALITY CONTROL
The control area (C) serves as an internal procedure control. A coloured line appears when the procedure or sample volume has been applied correctly. Control standards are not supplied with this test. As good laboratory practice, it is recommended that positive and negative controls be performed regularly to verify test performance.
RESTRICTIONS
• This test is intended for the qualitative detection of SARS-CoV-2 viral antigens only. The exact concentration of SARS-CoV-2 virus antigens cannot be determined in this test.
• Test results are for clinical reference only and should not be the sole basis for clinical diagnosis and treatment. Clinical management of patients should be considered in combination with their symptoms, physical signs, patient history, other laboratory tests, therapeutic responses and epidemiological information.
• Proper sample collection is crucial. Failure to follow the procedure can lead to inaccurate test results. Improper collection, storage or even freezing and thawing of the sample can lead to inaccurate test results.
• A false-negative test result may occur if the viral antigen level in a sample is below the detection limit of the test or if the sample was not collected or transported properly; therefore, a negative test result does not rule out the possibility of SARSCoV-2 infection.
• A positive result does not exclude co-infection with other pathogens.
• Monoclonal antibodies may not recognise SARS-CoV-2 viruses with slightly altered amino acid levels in the region of the target epitope, or may recognise them with less sensitivity.
• The amount of antigen in a sample may decrease with increasing disease duration. Samples taken after the 5th day of illness are more likely to be negative compared to an RTPCR test.
• The tests target the nucleocapsid proteins. Performance is not affected by mutations in the spike protein. Mutations in the nucleocapsid protein are not excluded in the future.
You can download these instructions here download
General FAQ
How does Odem ensure high quality at such a fair price?
The Better AG, founded in 2006 in Switzerland, has become the main supplier for thousands of companies in recent years.
The strategy:
• Bulk purchasing
• Close quality control of the goods
• Passing on the purchasing advantages to our customers
• By offering the possibility to receive free samples, our customers are not taking any risks.
What is the money-back guarantee?
If you are not satisfied with your goods, you can return them within 14 days of purchase and receive a full refund.
What payment options are available?
We offer a convenient payment by invoice after receipt of the goods to a German bank account.
Has the goods already been cleared through customs?
Yes, the goods have already been cleared through customs and will be delivered to you from our German warehouse. You will not incur any additional costs.
Product Sheet
Login