Covid Test
Covid test kits are essential for detecting and managing COVID-19 infections effectively. We strive to provide healthcare providers, clinics, and individuals with the best COVID-19 test kits. Accurate results and rapid testing empower timely medical decisions and enhance public health safety.
Recommended Solutions for Accurate COVID-19 Tests
We are pleased to continue providing you with Covid-19 Antigen Rapid Tests (Professional & At-home tests), Combo Test Kits and PCR Tests, so you can ensure timely and reliable COVID-19 detection.
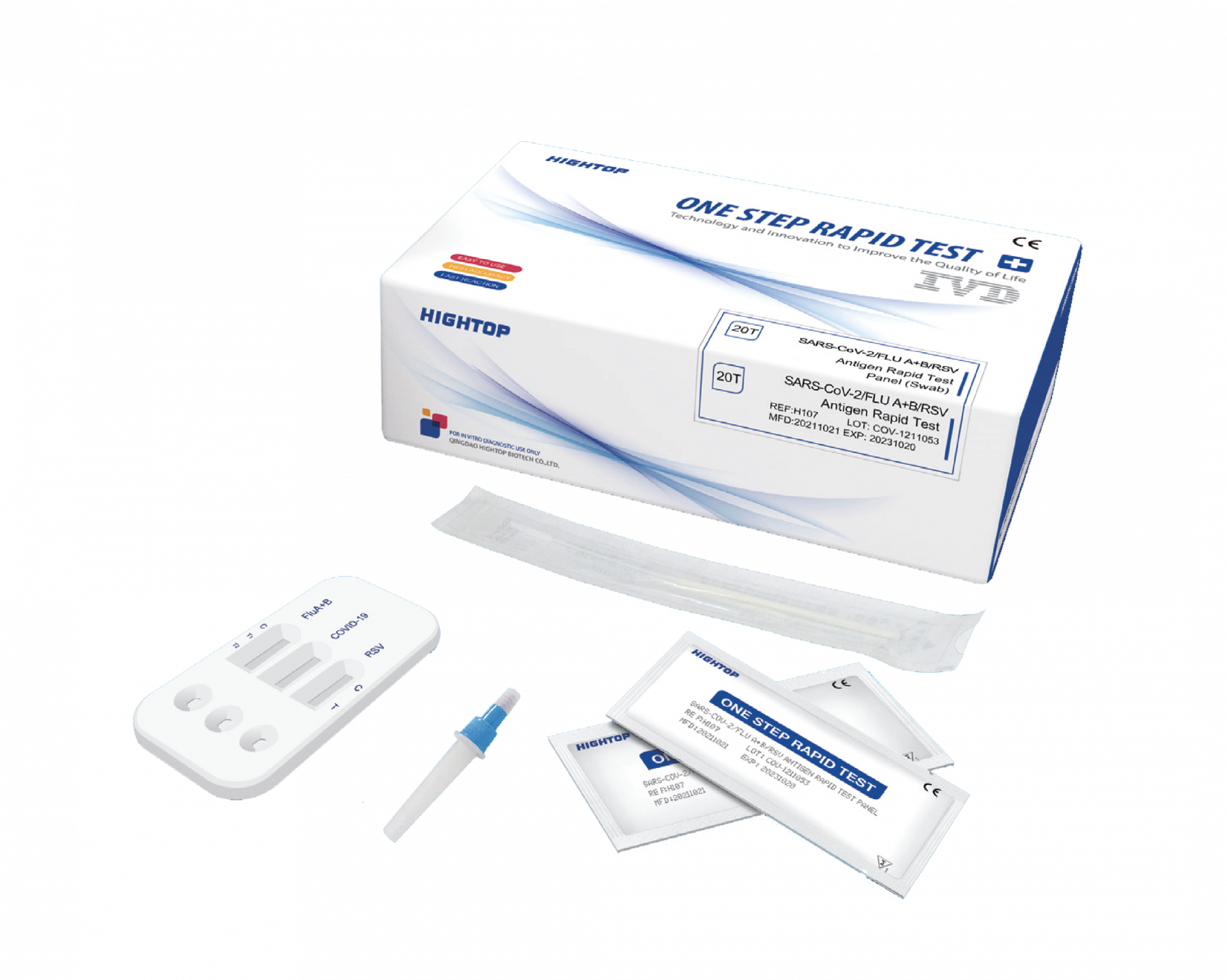
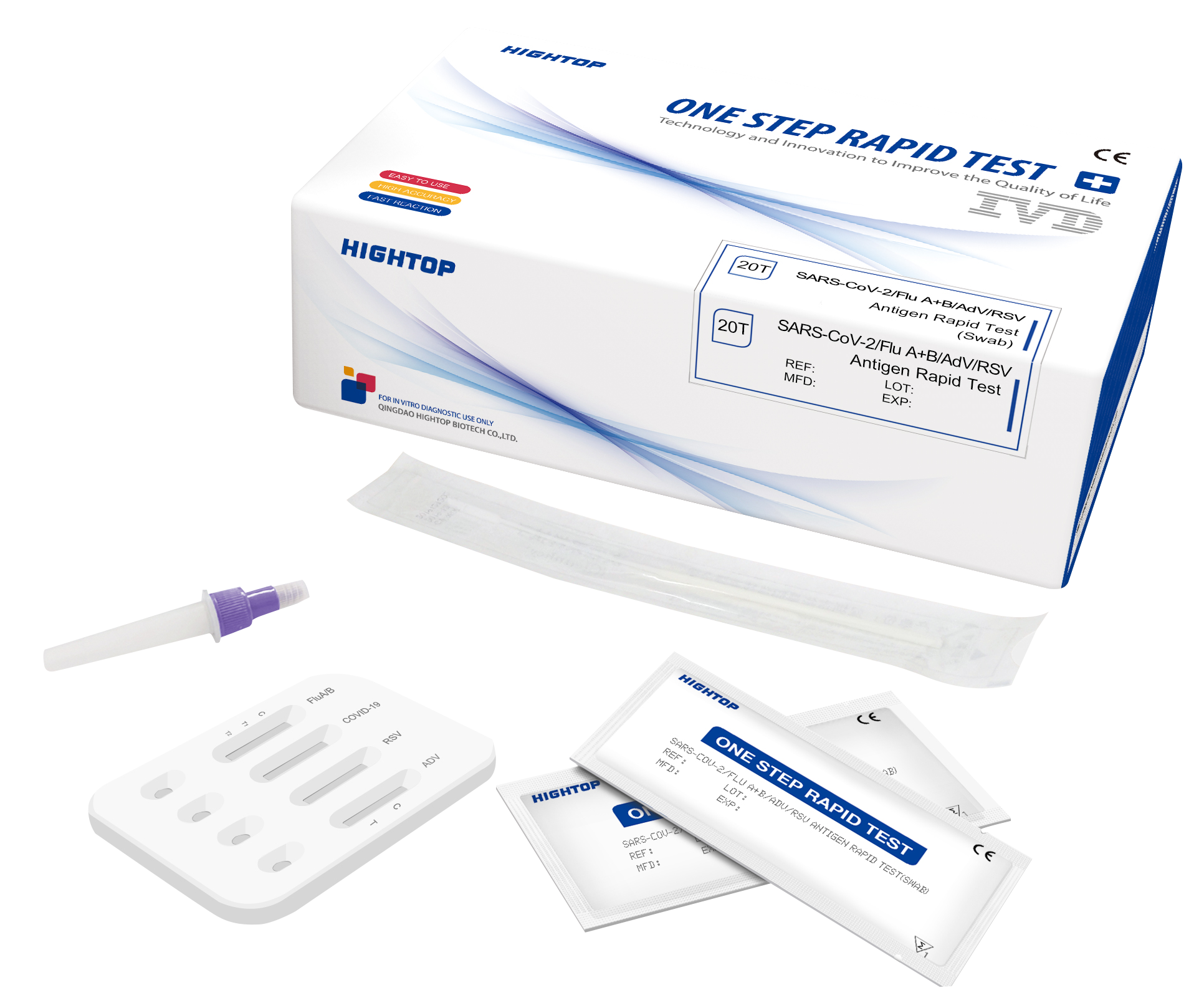
Hightop 4 in 1 (SARS-CoV-2 | Influenza A | Influenza B | RSV)
✓ Hightop 4in1 Test ✓ Buy Hightop 4in1 Test ✓ HighTop COVID-19, Flu A/B & RSV Test Kit ✓ SARS-CoV-2/FLU A+B/RSV ✓Order Hightop 4in1 test now!
View Product

Green Spring Covid Test Kit
Buy Green Spring SARS-CoV-2 antigen rapid test kit 4 in 1 online at OdemShop. Practical 4 in 1 antigen test: nasopharyngeal, pharyngeal, nasal or lollipop test
View Product

Fluorecare SARS-CoV-2 & Flu A/B & RSV Antigen Combo Self Test
Shop the Fluorecare 4 in 1 Combo Antigen Self Test Kit for SARS-CoV-2, Influenza A/B, and RSV. Get fast and easy results in just 15 mins. CE marked.
View Product
FAQ for COVID Test Kits - Covid Antigen Tests
COVID test kits are essential for detecting and managing Coronavirus infections. Typically, samples are taken via nasal swabs or saliva collection. The leading method is point-of-care rapid antigen testing. Find more details here on recommended usage, technical data, and why you should read the FAQ for step-by-step guidance and additional insights.
- What types of COVID test kits are available, and how do they differ?
- How can I evaluate the reliability of Covid Antigen Tests?
- How do COVID-19 Tests detect infections?
- Who should consider using SARS-CoV 2 antigen test kits?
- What is the accuracy of rapid antigen tests?
- What does a Coronavirus positive test result mean?
- Why are Covid-19 diagnostic tests crucial for public health?
- Do you offer other infectious disease tests and a ranking of the best?
- Where can I find a comprehensive comparison of COVID test kits?
What Are the Differences Between Corona Diagnostic Testing Types?
COVID tests can be categorized into rapid tests and laboratory-based evaluations. Some tests detect past infections (antibody tests), while others identify current infections (antigen and PCR tests).
Antigen Tests
Antigen tests are quick and help identify current infections by detecting specific viral proteins. They are available for both professional and at-home use. While convenient, they have lower sensitivity than some lab-based tests, which can result in false negatives or positives.
Antibody Tests
These tests reveal if someone has had a past infection by identifying the presence of antibodies. They are not suitable for diagnosing current infections but can indicate previous exposure to the virus.
PCR Rapid Tests
PCR rapid tests share similarities with traditional PCR tests but are processed on-site for quicker results. They involve collecting samples from the throat or nose and provide accurate results within hours, though slightly less sensitive than full PCR tests.
PCR Test (RT-PCR)
Samples are collected using a swab from the mouth, nose, or throat and analyzed in a lab. Results are typically available within 24 hours. Known for its high sensitivity and specificity, the PCR test is often regarded as the "gold standard" for detecting COVID-19 by identifying the virus's genetic material.
How Can I Assess the Accuracy of COVID Testing Kits?
To evaluate the accuracy of COVID testing kits, check for FDA or CE approval, review sensitivity and specificity data, consult independent expert studies , and verify inclusion on the EU Common List for rapid tests.
How Do COVID-19 Tests Identify Infections?
COVID-19 tests identify infections by detecting viral proteins or genetic material. Antigen tests find proteins from the virus that causes covid-19, while PCR tests detect its RNA, both indicating active infection.
Who Should Consider Using SARS-CoV-2 Antigen Test Kits?
Individuals needing rapid results, such as those with symptoms, close contacts of confirmed cases, or those in high-risk environments, should consider using SARS-CoV-2 antigen test kits for quick detection.
What Is the Accuracy of Rapid Antigen Tests?
Rapid antigen tests generally offer high specificity but moderate sensitivity. They are most accurate when viral load is high, such as early in infection or when symptoms are present.
What Does a Positive COVID-19 Test Result Mean for At-Home COVID-19 Antigen Test?
A positive result from an at-home COVID-19 antigen test indicates the presence of the virus, suggesting an active infection. Follow public health guidelines for isolation and notifying contacts.
Why Are COVID-19 Diagnostic Tests Crucial for Public Health?
COVID-19 diagnostic tests, including OTC COVID-19 antigen diagnostic tests, are crucial for public health as they provide quick results to identify and isolate infections. Accurate COVID-19 antigen diagnostic test results help control virus spread and guide health measures.
Do You Offer Other Infectious Disease Tests, and Is There a Ranking of the Best?
Yes, we do offer more infectious disease tests :
Influenza Test
Best Product:Sejoy
3in1 SARS-CoV-2 | Influenza A | Influenza B Test
kit
Source:Best
Influenza Test Kits
RSV Test
Best Product: Cordx 4in1
Self-Test (RSV | Influenza A/B | SARS-CoV-2)
Source: Best RSV Tests
Combo Test
Best Product: Sejoy
SARS-CoV-2 influenza a+b RSV antigen 4 in 1 combo rapid Test
Source: Best
Combo test kits
Where can I find a comprehensive comparison of COVID test kits?
You can access a comprehensive comparison of COVID test kits here. This resource provides detailed insights to help you choose the most suitable option for your needs.